Hydrogen Sulfide Removal: Effective and Sustainable Strategies for Industries
1. Introduction to Hydrogen Sulfide Removal
Hydrogen sulfide (H2S), known for its characteristic rotten egg smell, is one of the most dangerous gases in industrial environments. This byproduct, found in activities such as mining, wastewater treatment, and oil refining, not only presents risks of toxicity and corrosion, but also challenges operational efficiency and equipment integrity.
2. Understanding the Impact of H2S
Health and Safety Impacts
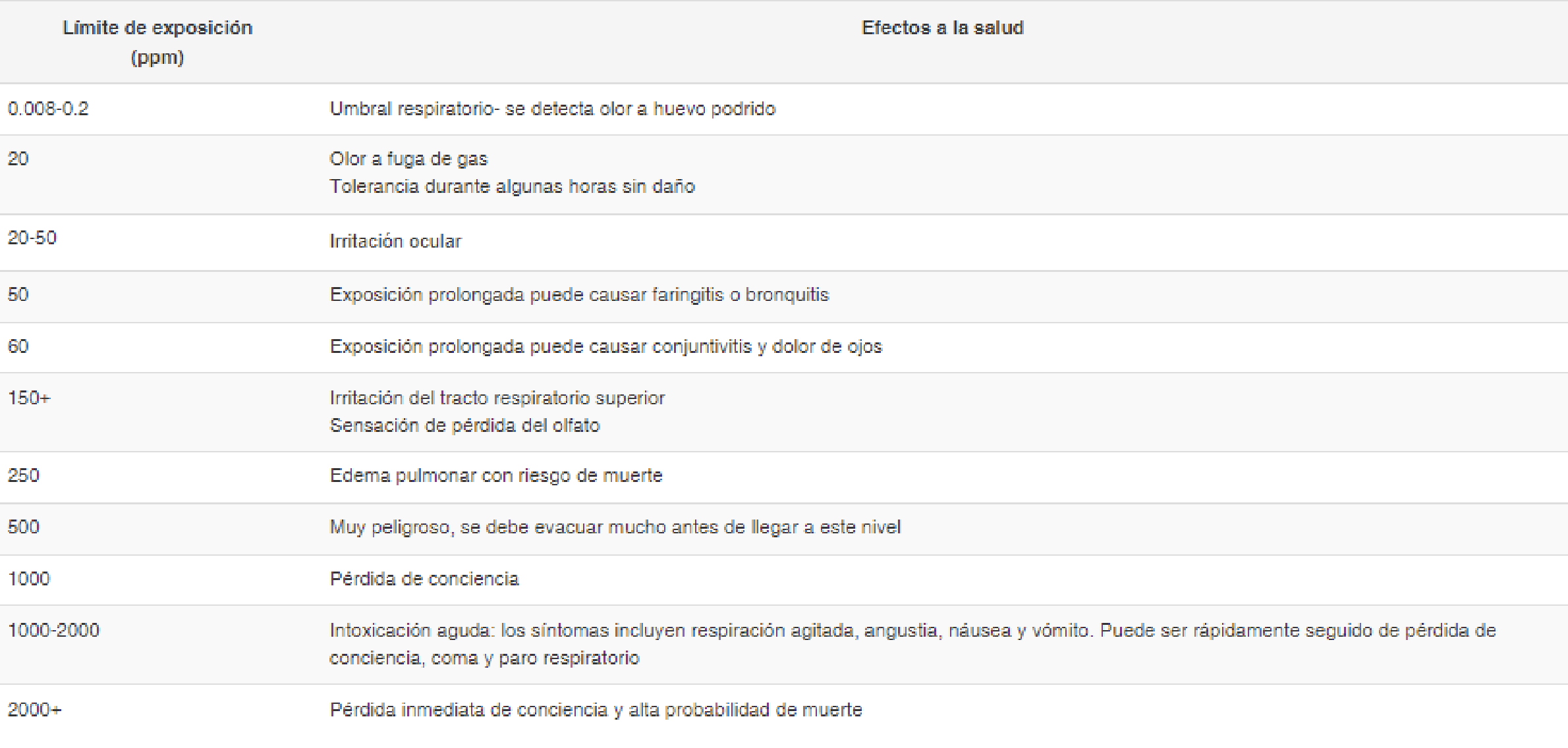
Exposure to H2S can have serious health consequences, varying according to concentration:
 Environmental Impacts
Environmental Impacts
H2S contributes to acid rain, eutrophication, soil and water acidification, and biodiversity reduction. Notable examples include the anoxia in the depths of the Black Sea.
3. Methods for Hydrogen Sulfide Removal
Chemical Methods
- Oxidation: Using oxygen, ozone, chlorine, or hydrogen peroxide to convert H2S into elemental sulfur or sulfate.
- Adsorption: Application of activated carbon, zeolites, iron compounds or alumina to trap H2S.
- Absorption: Using water, amines, or alkaline solutions to dissolve H2S.
Physical Methods
- Condensation: Cooling or compressing to liquefy and separate H2S from gas.
- Membranes: Selective barriers to filter H2S.
- Ion Exchange: Resins that exchange sulfide ions.
Biological Methods
- Biofiltration: Bacteria in organic or inorganic beds oxidizing H2S.
- Bioreactors: Microorganisms in a closed reactor to oxidize H2S.
- Biodesulfurization: Anaerobic process to reduce sulfate to sulfide.
4. The Role of Iron Compounds
Iron compounds can react with H2S by various reaction pathways.Iron compounds can be natural, like iron minerals, or synthetic, like iron oxides produced by oxidizing scrap metal. Advantages of this method of desulphurisationinclude:
- Simple and economical method, not requiring complex equipment.
- Environmentally friendly, generating no toxic waste or pollutant emissions.
However, this method also has limitations, such as:
- The availability and cost of iron compounds may vary depending on the source and quality.
- The reaction may be slow or incomplete, depending on temperature, pressure, pH, and H2S concentration conditions.
- The reaction may generate undesirable by-products, like hydrochloric acid when iron chloride is used, causing corrosion or scaling in equipment.
5. Safety Considerations and Regulations
H2S is a very toxic gas and corrosive, which can cause significant health safety problems, corrosion of metallic elements in structures as well as environmental problems due to its uncontrolled atmospheric release. It is also a flammable gas if levels are higher than 4%, that can cause explosions or fires in the presence of an ignition source. For these reasons, handling H2S requires specific safety measures and regulations, such as:
- Using appropriate personal protective equipment, like masks, gloves, glasses, and resistant clothing.
- Using H2S detection and alarm systems to alert to the presence and concentration of the gas in the environment.
- Using ventilation and extraction systems to prevent the gas’s accumulation and dispersion in the work area.
- Complying with local and international standards on H2S emission and exposure limits, which vary depending on the sector and country. Some examples include:
- The World Health Organization (WHO) recommends an exposure limit of 10 ppm for 8 hours a day and 40 hours a week.
- The United States Occupational Safety and Health Administration (OSHA) sets an exposure limit of 20 ppm for 10 minutes and an action limit of 10 ppm for 8 hours a day and 40 hours a week.
- The United States Environmental Protection Agency (EPA) sets an emission limit of 0.13 kg of H2S per ton of processed sulfur in sulfur recovery plants.
- The Spanish National Institute for Safety and Health at Work (INSST) sets an exposure limit of 10 ppm for an exposure of 8 hours per day and 40 hours per week.
6. Impact of Effective H2S Removal
Effective H2S removal means a significant reduction in the risks and costs associated with this gas, translating into long-term benefits for businesses. Some of these benefits include:
- Improved safety and health for workers and nearby communities, avoiding accidents, illnesses, and discomfort caused by H2S.
- Improved quality and value of the final product, eliminating H2S’s unpleasant odor, as well as impurities that can affect its performance.
- Improved process efficiency and productivity, avoiding corrosion, scaling, blockages, and damage to equipment caused by H2S.
- Improved reputation and social responsibility for companies, complying with environmental standards and demonstrating their commitment to sustainable development.
7. Frequently Asked Questions (FAQs)
Hydrogen Sulfide Removal Frequently Asked Questions (FAQs)
How to remove hydrogen sulfide?
Hydrogen sulfide removal can be done using various methods, depending on the context and gas concentration. Common techniques include:
- Chemical Methods: Like oxidation with oxygen, ozone, or hydrogen peroxide, converting H2S into elemental sulfur or sulfates.
- Adsorption: Using activated carbon or zeolites to trap H2S on their surface.
- Absorption: Using chemical solutions like amines or carbonates to dissolve H2S.
- Biological Methods: Using biofiltration or bioreactors where microorganisms degrade H2S.
- Iron Compounds: Reacting H2S with iron compounds to form insoluble iron sulfides.
How to eliminate sulfide?
The term “sulfide” encompasses various compounds containing sulfur, including hydrogen sulfide. Removing sulfides generally involves chemical or biological processes similar to those used for H2S, adapted to the specific nature of the sulfide compound in question.
How dangerous is hydrogen sulfide?
Hydrogen sulfide is extremely dangerous and toxic. Even at low concentrations, it can cause irritation in the eyes and respiratory system, and at higher levels, it can result in loss of consciousness, brain damage, coma, or even death. It is also corrosive to metals and can cause failures in industrial equipment.
How to prevent and controlhydrogen sulfide?
To prevent and control the formation or accumulation of hydrogen sulfide, several measures can be taken:
- Process Control: Modify industrial processes to minimize H2S production.
- Adequate Ventilation: Ensure good ventilation in areas where H2S may be generated.
- Monitoring and Detection: Install H2S detectors in areas prone to its accumulation.
- Use of Inhibitors: Apply chemicals that inhibit H2S formation in industrial processes.
- Regular Maintenance: Inspect and maintain equipment to prevent H2S leaks or accumulations.